Best Practices for Investigations & Continuous Improvement: CAPA, Complaints, NCRs, etc.
Quality Environment
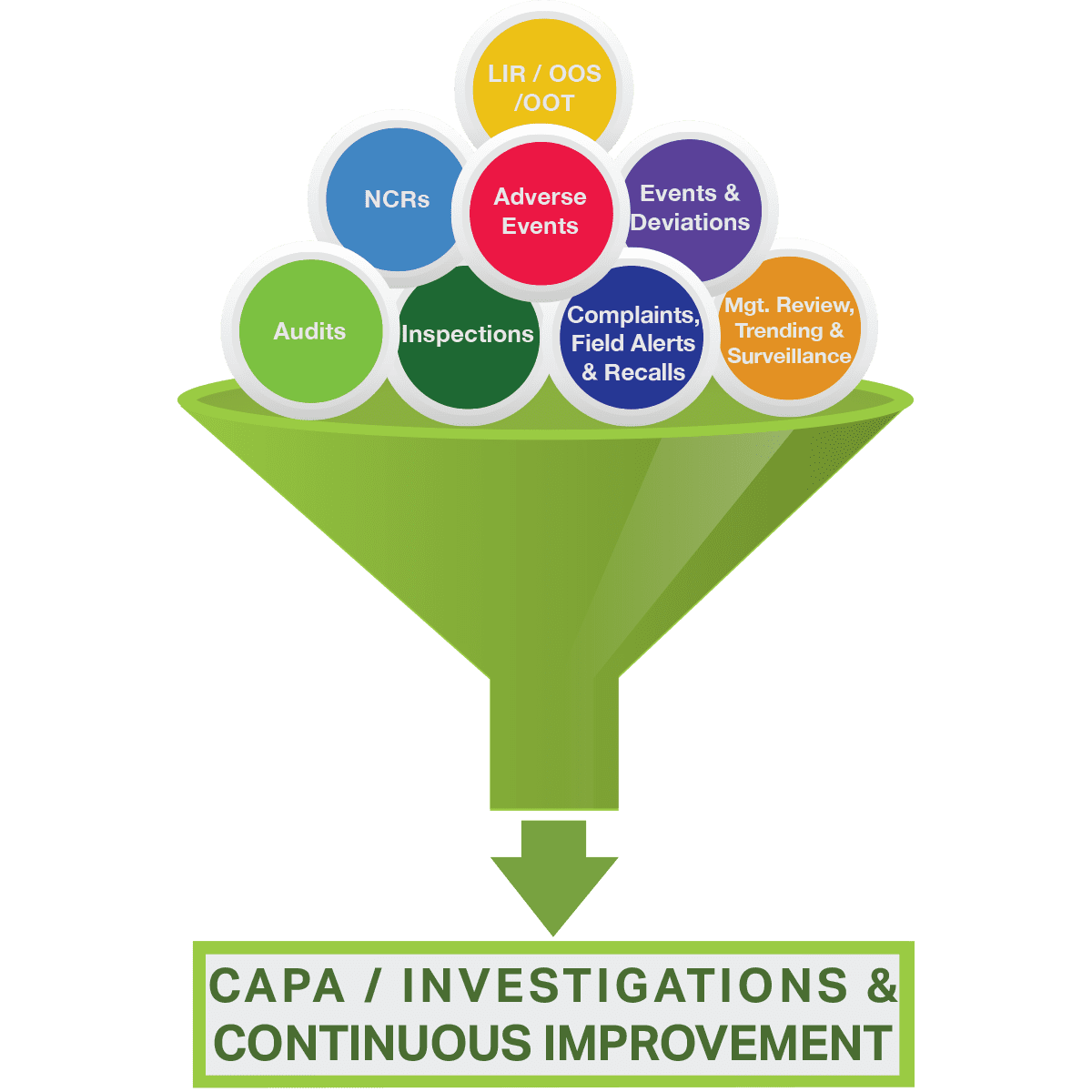
Frequent CAPA
Investigation System Issues
Investigation System Issues
Best Practices
Why CAPA FOR HIRE?
Pharmaceutical Quality: The Current Environment
-
- Increased global competition with growing regulatory and economic pressure
- Drug Shortages highlighting trade-offs between Public Health, Quality, and Sales
- Increased User Fee Funding Promises More Challenging FDA Inspections
– FDA National Experts are raising the bar for aseptic practices, environ. monitoring, etc.
– Observations are escalating more frequently/quickly to Warning Letters
– FDASIA/GDUFA is driving greater parity in foreign vs. U.S. inspections - Legacy processes, systems, equipment, and facilities raise the level of risk
- Pressure to reduce costs and achieve growth objectives may cause:
– Quality System gaps and overlaps (e.g. poor post-merger integration)
– Cultural disconnects in balancing Quality and Production objectives
– Loss of organizational knowledge (buyouts, outsourcing, retirement packages)
– Inability to keep up (e.g. backlogs in AEs, CAPAs, product complaints, NCRs, OOS)
Industry Faces Unprecedented Economic and Compliance Pressure
Why Does CAPA Matter?
- Public Health depends upon access to quality drug products
- Life Sciences Companies conform to cGMP requirements to assure the identity, strength, quality, and purity of drug products
- Effective CAPA Systems demonstrate a commitment to accountability and Continuous Improvement
- CAPA (Corrective and Preventive Actions) related items continue to feature prominently in FDA inspectional findings
- The CAPA System provides a “Vital Signs” view across Quality Systems and is critical for identifying and remediating Quality System deficiencies
Structured CAPA Systems (including a Culture of Continuous Improvement) are Critical for Compliance AND Sustainable Competitive Advantage.
Common CAPA / Investigation Related Deficiencies
- Aging backlogs of deviations, complaints, CAPA, etc.
- Leadership may not understand or demonstrate a commitment to CAPA
- Organizational culture may tolerate repeat mistakes with limited focus on accountability, learning and Continuous Improvement
- Management is often asked to do more work with less resources (often lack capacity and/or capability), and CMOs are often neglected
- Oversight is often non-strategic (e.g. not prioritized or risk-based)
- Firms not operating within a “state of control” placing public health at risk
- Root cause analysis may be limited (e.g. blame placed on “human error” or “procedure not followed”), corrective actions may be incomplete, and “effectiveness checks” may be neglected
- Staff may be enamored by tools & technologies vs. focusing on risk-based CAPA closure
- Information gathering may not occur at the time of the incident
- Subject Matter Experts (SMEs) are not engaged
- Processes and systems may be complex and contradictory

Best Practices for CAPA & Continuous Improvement
- A culture of Continuous Improvement, learning, accountability, and reporting without retaliation
- Leadership engagement & support
- Patient-focused Risk Based Prioritization
- Practical Management Controls & Metrics
- Timely completion of investigation activities
- Team-based approach with access to SMEs
- Communication between CAPA and other Quality Systems
- Fit for purpose use of tools and techniques with effort linked to priority/risk
- Training/certification of core Investigation Team members with thorough SOPs (Investigation Plan, roles, etc.)
- Practical Performance Management

OUR SERVICE OFFERINGS FOR OBTAINING TRANSFORMATIONAL GAINS IN CAPA SYSTEMS INCLUDES:
- High level assessment to Establish Baseline* and design the preliminary strategy
- CAPA related systems audit (checklist driven; needs based)
- Project Mgt. & Change Mgt. support (Project Plan, Communications Plan, staff engagement, management controls/metrics, cultural strategy, etc.)
- Risk Based Prioritization of open investigations (e.g. Complaints, CAPA, NCRs, Deviations, OOS, etc.)
- Implementation support for optimizing CAPA related systems (processes, documentation/procedures, organizational design, IT workflow, metrics, etc.)
- Interim Subject Matter Expert Support to “dive-in” and use teamwork to complete high risk investigations while training and coaching internal staff (as appropriate)
- On-the Job Training (OJT) of designated staff with focus on eliminating backlogs
- Coordinated transition to your team to enable sustainable transformational gains
- Periodic mentoring and monitoring of metrics and report quality
The Project Approach is Tailored to each Client’s Unique Needs
*detailed assessment to be performed based on extent of issues across organization, process, & technology
Why capa for hire?
- We respect your time & money and tailor the approach based on your needs (e.g. interim support to eliminate backlogs and provide On-the Job Training, coaching, mentoring)
- We have deep experience in partnering with industry leading clients to understand their needs and implement transformational, sustainable gains in performance
- Our comprehensive business perspective with more than 20 years of industry experience across Quality, Manufacturing, Clinical Development, Regulatory, Pharmacovigilance, and Sales & Marketing sets us apart by understanding “how the pieces fit together” and in anticipating both opportunities and obstacles
- We have extensive experience in managing Transformational Business Improvement Projects including: Business/Lean Process Redesign, Organizational Design/Development, Technology Optimization, Change Management, Continuous Improvement, etc.
- Projects are carefully designed and managed to engage stakeholders at all levels; driving sustainable performance improvement through effective transition planning
- We are relentless in defining, communicating, and achieving a shared vision through teamwork
- Our unique business model brings senior level experienced practitioners with the right skills at the right time at a fair value proposition








CAPA FOR HIRE IS A SERVICE OFFERING FROM SPLASH CONSULTING
Please use the form below or call: 603.479.1068
Module ID attribute is required!
